In order to appreciate architectural coatings that reduce heating and cooling costs, it is important to understand the fundamentals regarding US energy consumption. According to the U.S. Energy Information Service, 40 percent of all US energy consumption is used for heating and cooling residential and commercial buildings. For homeowners, 25 percent of their average energy bill is for cooling. Considering these facts, consumers appreciate any efficiencies coatings formulators can offer.
Heat transfer mechanisms
Prior to considering how coatings can be engineered to save heating and cooling costs, it is instructive to examine heat transfer mechanisms: radiation, conduction, and convection.
Radiation
As figure 1 indicates, radiation is the emission and propagation of light energy in the form of rays or waves through space:
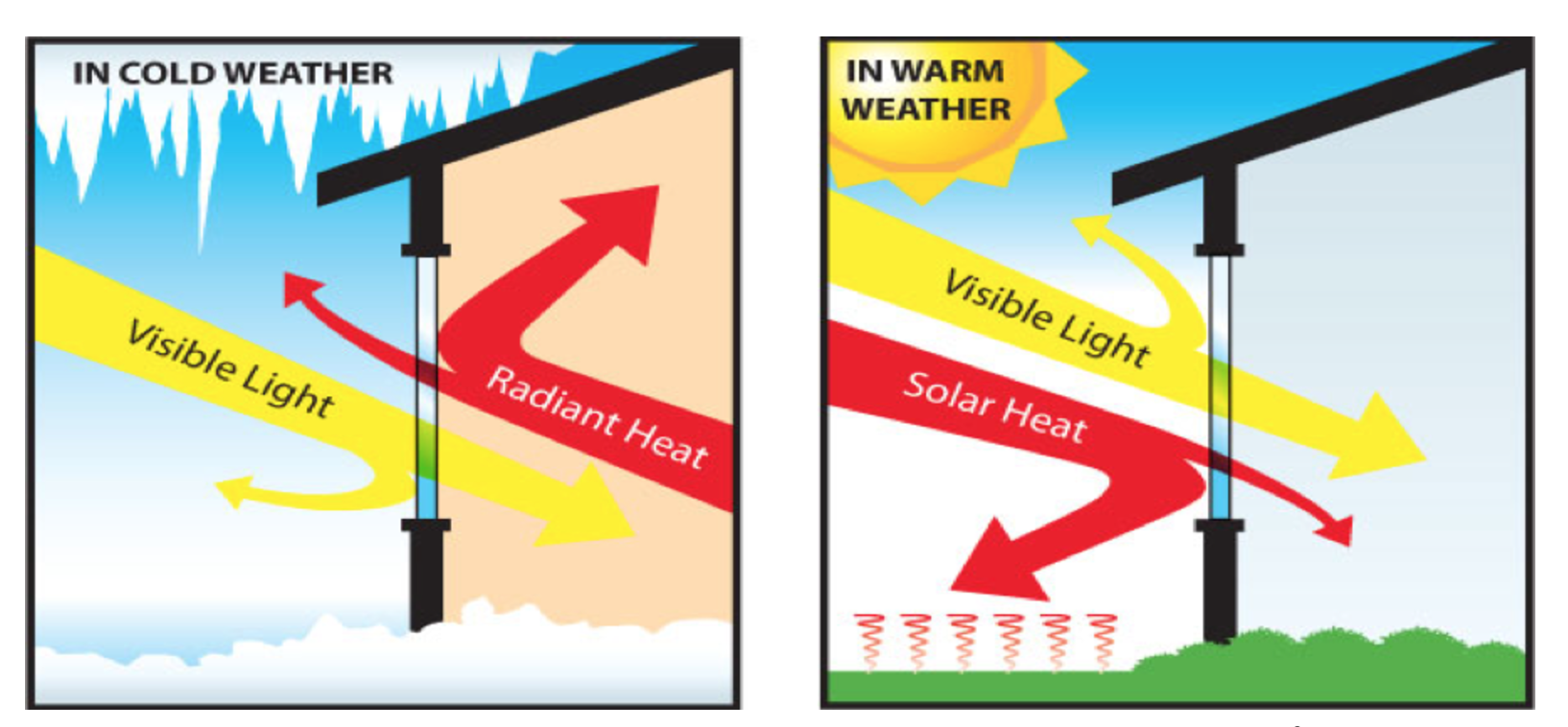
As figure 2 illustrates, pigments can absorb or reflect solar infrared energy based on their color. For example, if the pigment absorbs infrared (IR) energy (such as conventional darker pigments), we see heat build-up of the coated substrate. If the pigment reflects IR light (such as white and lighter colors), we see a lower increase in temperature.
To illustrate, the surface of a steel building at an ambient air temperature of 20° C will remain at about 20° C when painted white, whereas the surface will be about 35° C when painted black.
To read the rest of the article please click here to head over to UL Prospector.
__________
Ron Lewarchik, Author of article & President of Chemical Dynamics
As a contributing writer, Ron pens articles on topics relevant to formulators in the coatings industry. He also serves as a consultant for the Prospector materials search engine, advising on issues related to optimization and organization materials within the database.