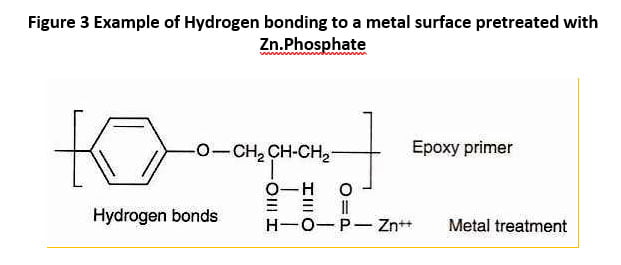
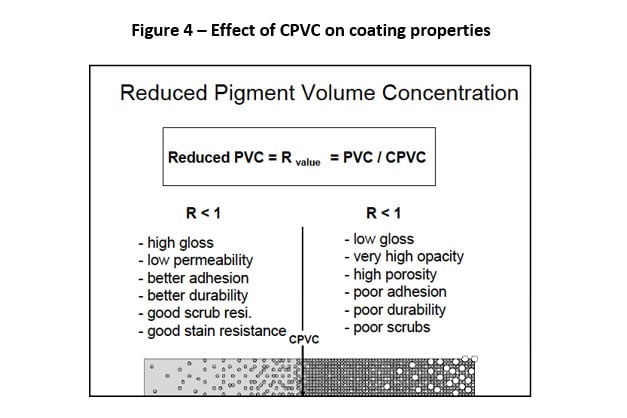
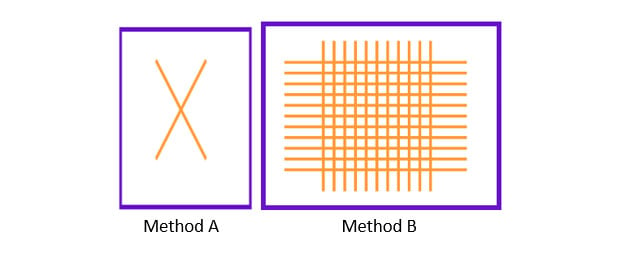
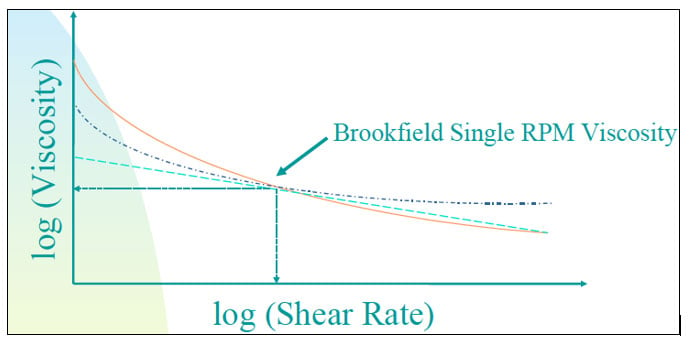
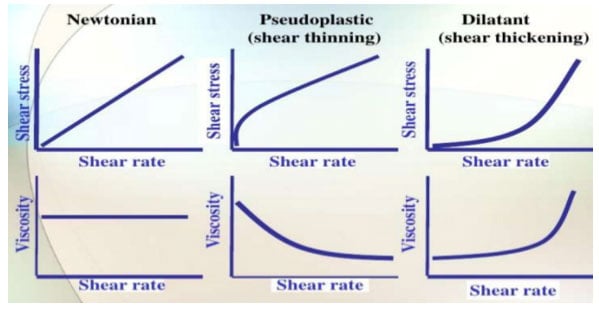
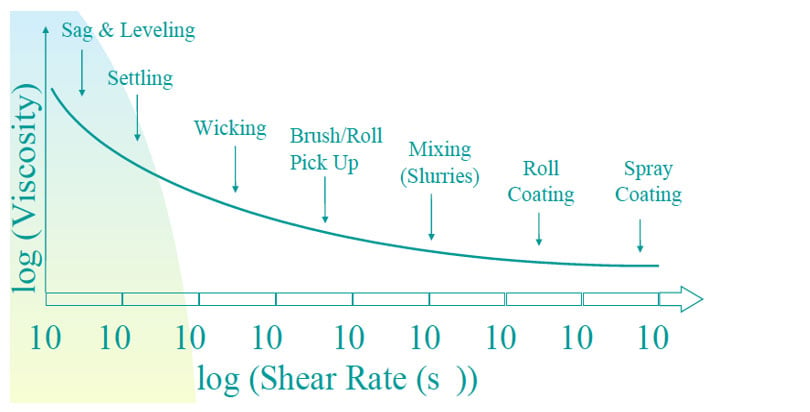
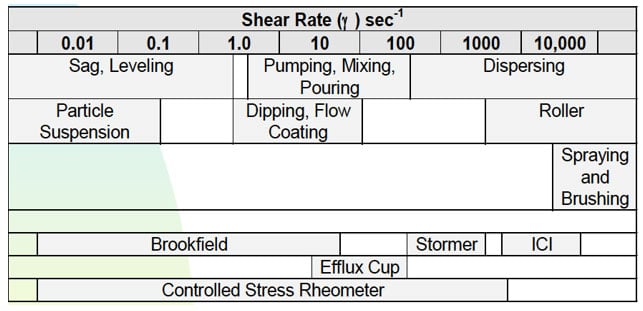
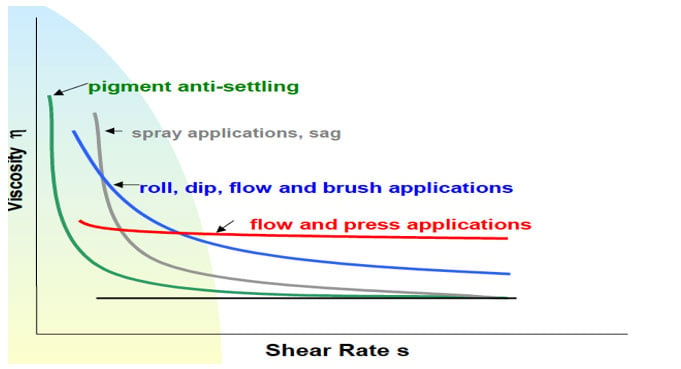
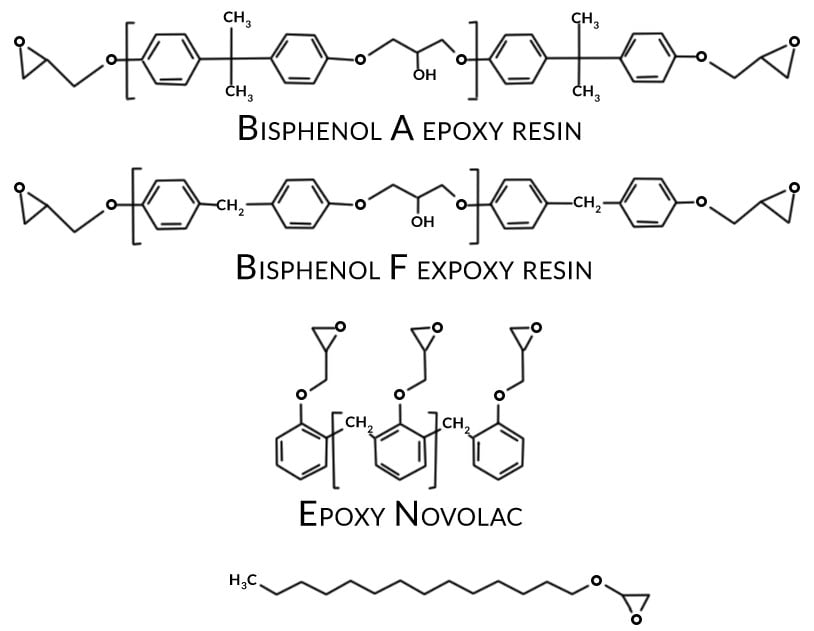
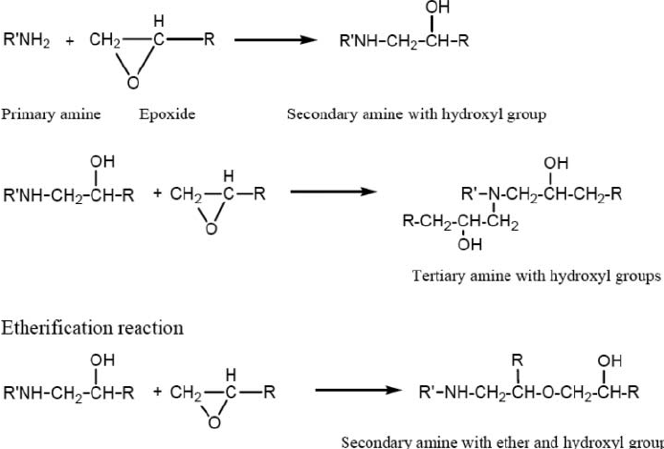
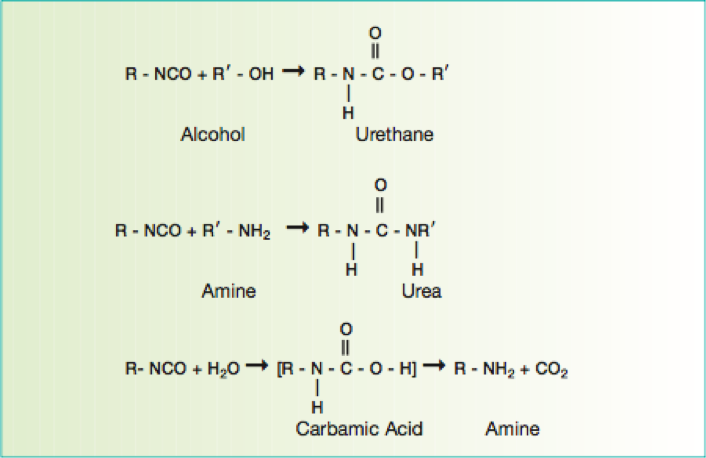
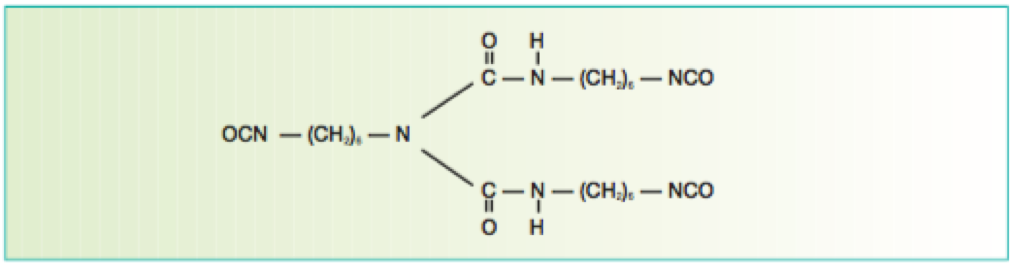
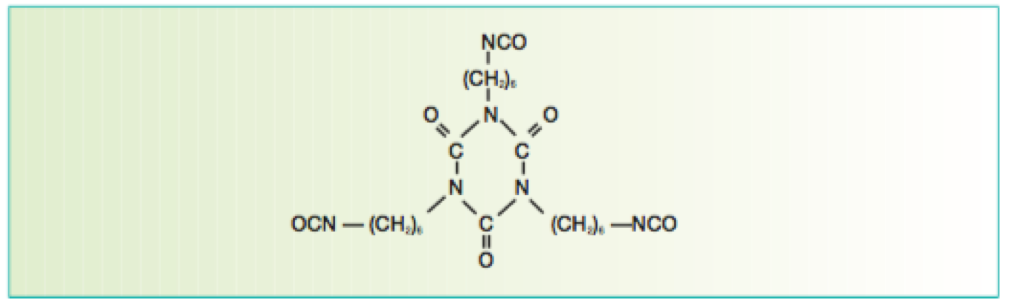
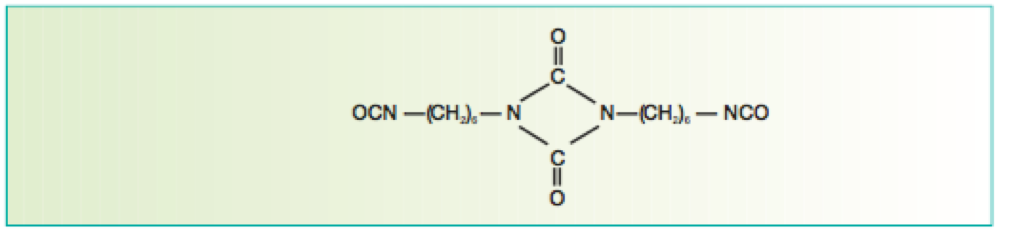
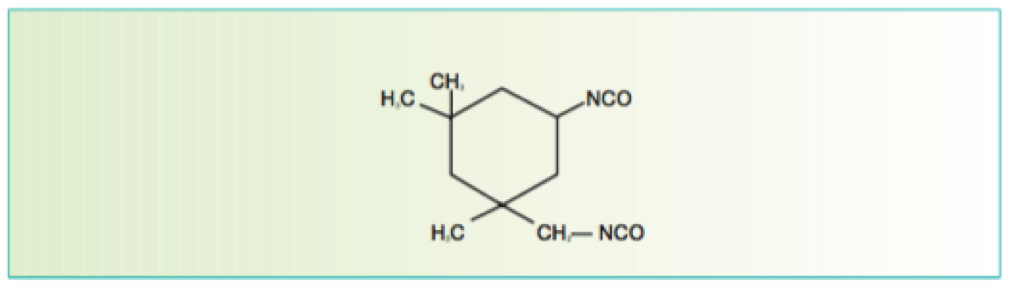
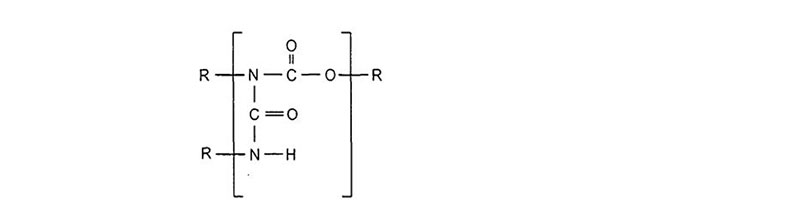
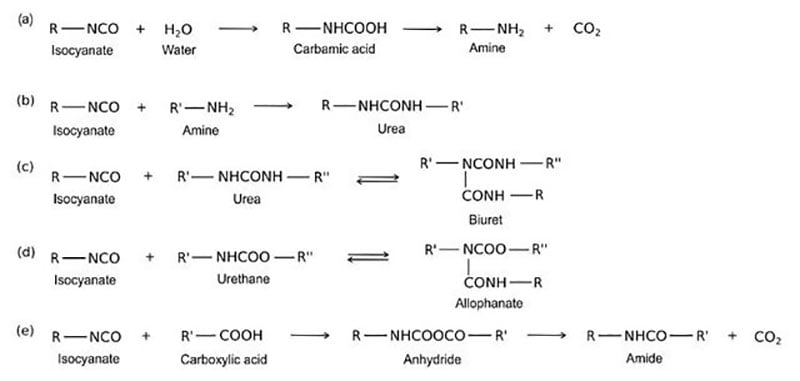
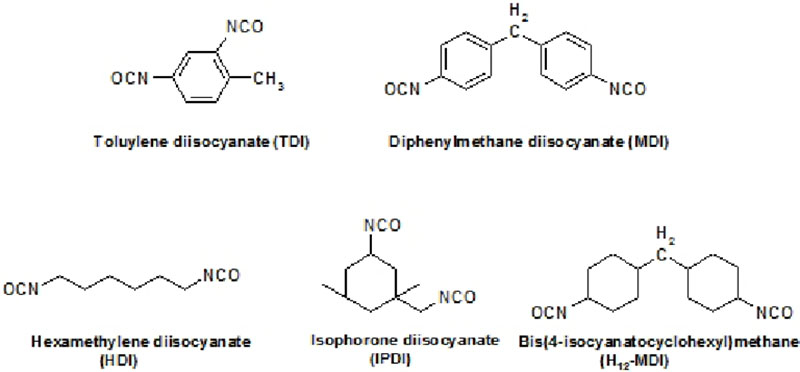
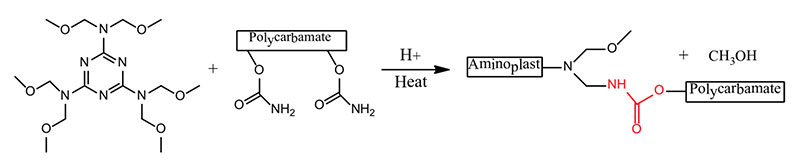
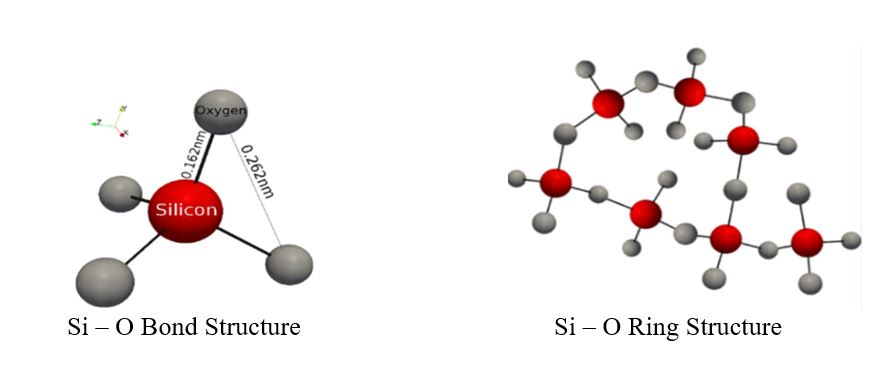
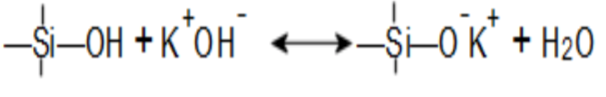
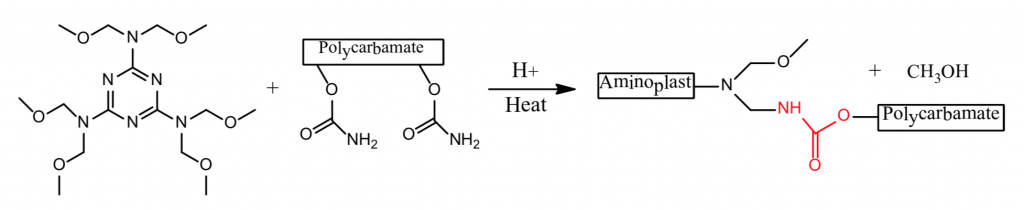
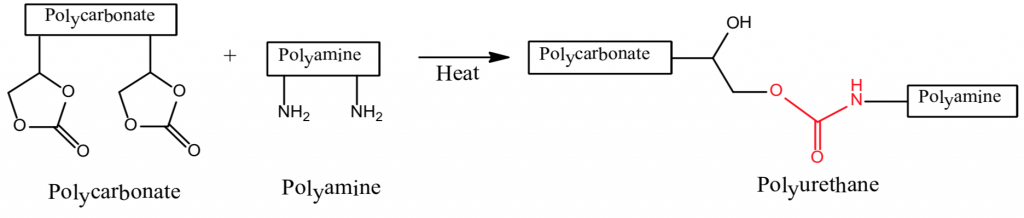
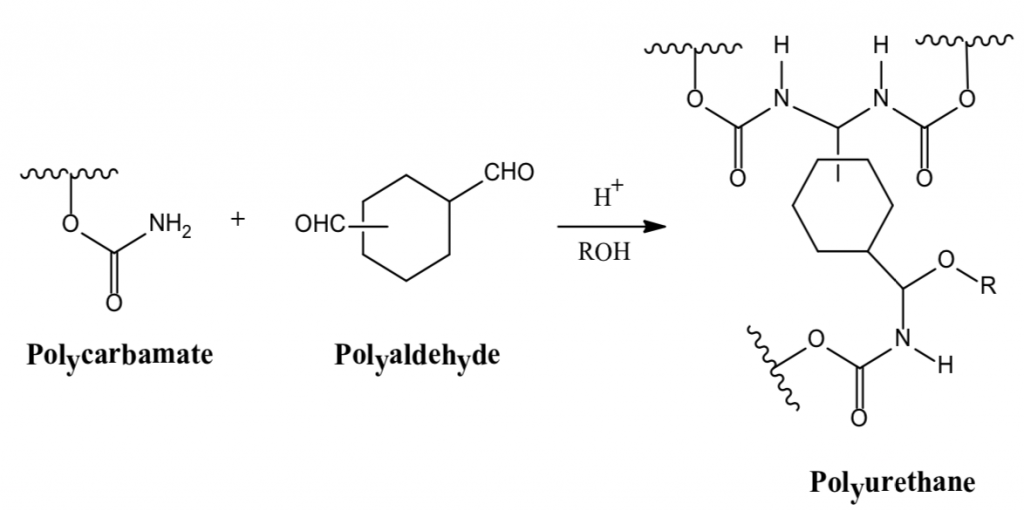
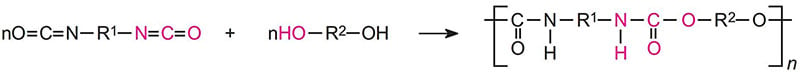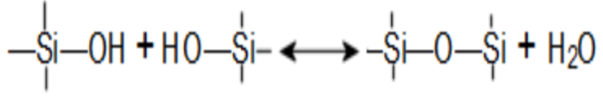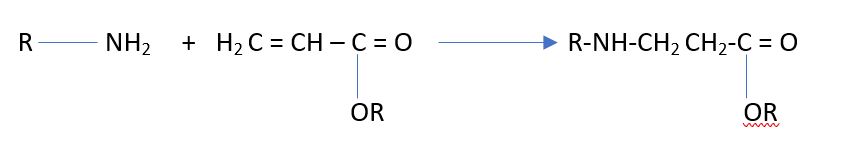There are many definitions for Smart Coatings, however they all have the common trait of being able to sense and interact with their environment. Smart coatings offer additional functional value to that provided by traditional properties of protection and decoration. A report by Transparency Market Research predicts the global smart coatings market will expand at a compound annual growth rate of 29.8% during the period between 2017 and 2025 and reach 1 billion dollars in sales by 2024.
External stimuli in smart coatings
External stimuli in smart coatings may include properties such as:
- Anticorrosion
- Antifingerprinting
- Antifouling
- Antimicrobiological
- Antifungal
- Color-shifting
- Easy clean
- Electrochromic
- Hydrophobic
- Hydrophilic
- Ice-phobic
- Photovoltaic
- Piezoelectric
- Piezo-magnetic
- Self-healing
- Solar-reflective
- Super-hydrophobic
- Thermochromic
These coating properties can be obtained by the use of novel specialty additives, pigments and/or polymers.
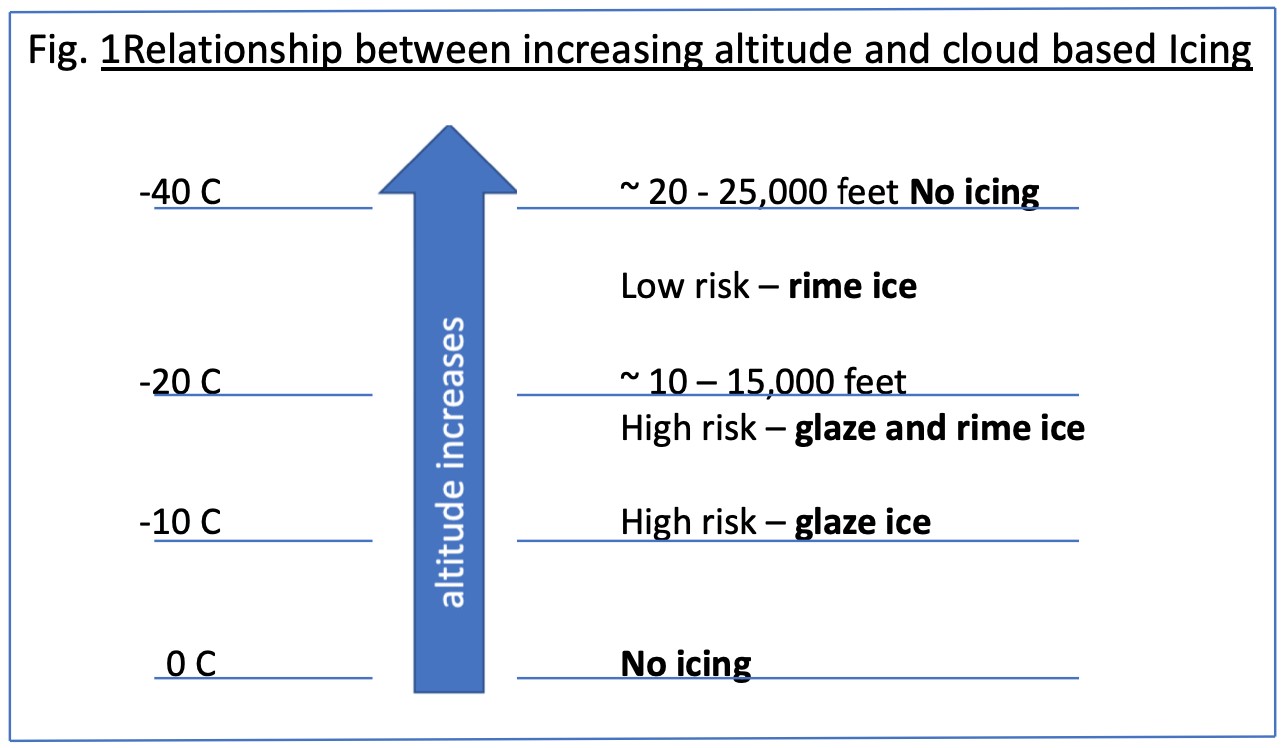
Icephobic coatings either resist the formation of ice on the surface to which ice has poor adhesion or facilitate the release of ice that has formed on the surface. Icephobic coatings have application in the aircraft industry, wind turbines and power lines. There are two types of ice formation that are problematic.
- Rime ice, more commonly known as frost
- Glare ice, more commonly called glaze ice, which forms a continuous layer of liquid water which freezes on the surface. Glare ice is particularly dangerous on power lines and aircraft.
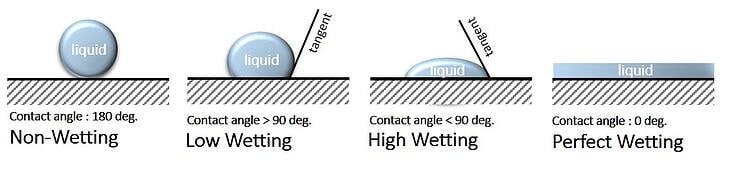
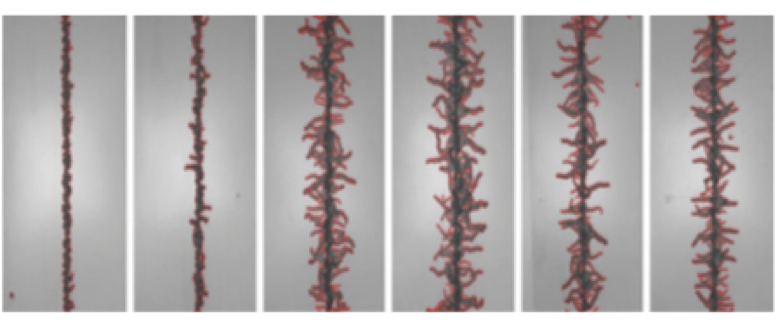
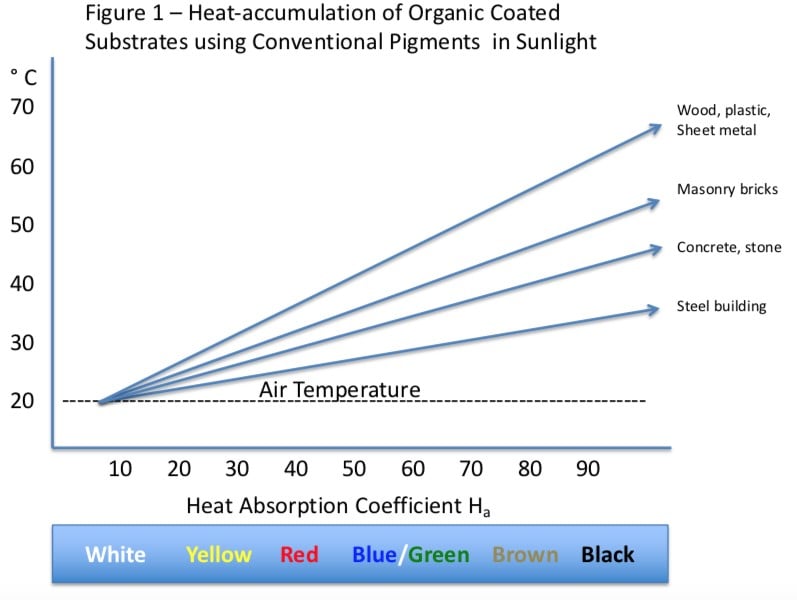
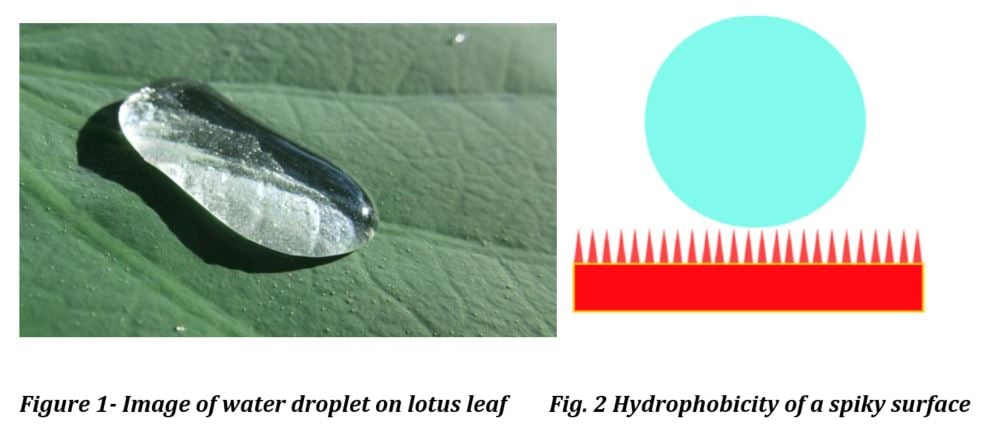
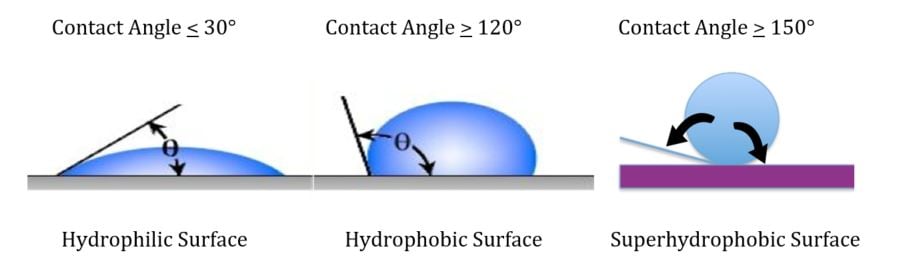
An icephobic coating can either be formulated to work for rime ice or glare ice, but not both. For Glare Ice some degree of hydrophobicity is necessary, however the surface structure of many superhydrophobic coatings can actually enhance ice adhesion. The low surface polarity and surface structure of superhydrophobic coatings renders the surface less icephobic than would be expected based on the contact angle. Figure 1 illustrates.
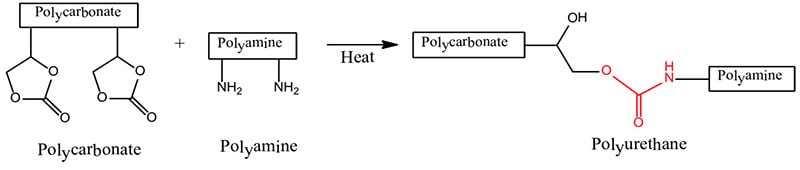
Some studies show that elastomeric polyurethane coatings provide less ice adhesion than that of coatings that are similarly structured but more glassy in nature. The theory is that the surface of the PU elastomeric coating induces slippage between the solid ice and that of the lightly cross-linked PU or silicone elastomeric structure with dangling chains at the surface.
Other approaches utilize freezing point depression on some surfaces or the addition of oils to low surface tension coatings. Lastly, some coatings utilize additives to increase the degree of undercooling required for ice nucleation to form.
Self-Healing Coatings
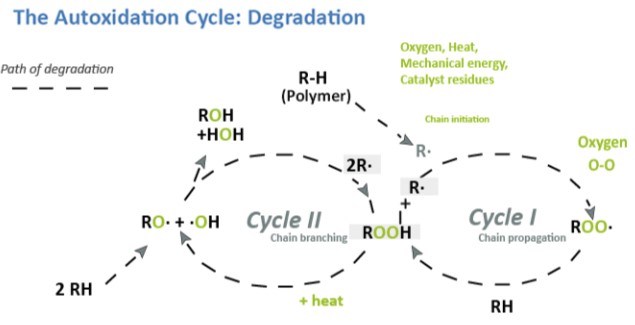
All coatings are susceptible to scratching and abrasion during their service life. Scratching and abrasion not only has an adverse effect on appearance, but further reduce the effective life expectancy in the event that the coating is applied over an oxidizable metal surface.
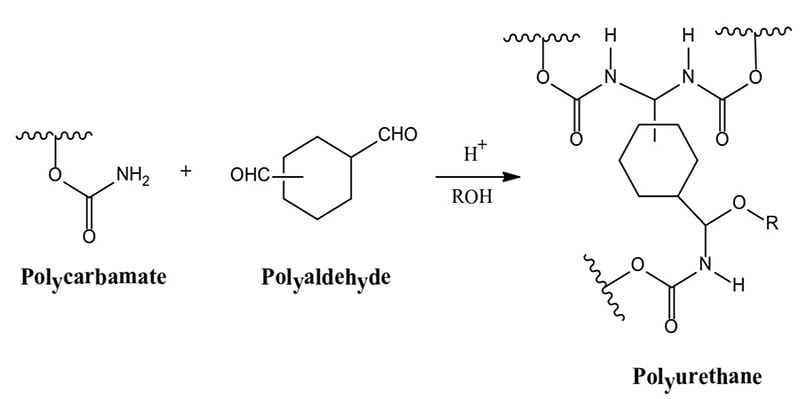
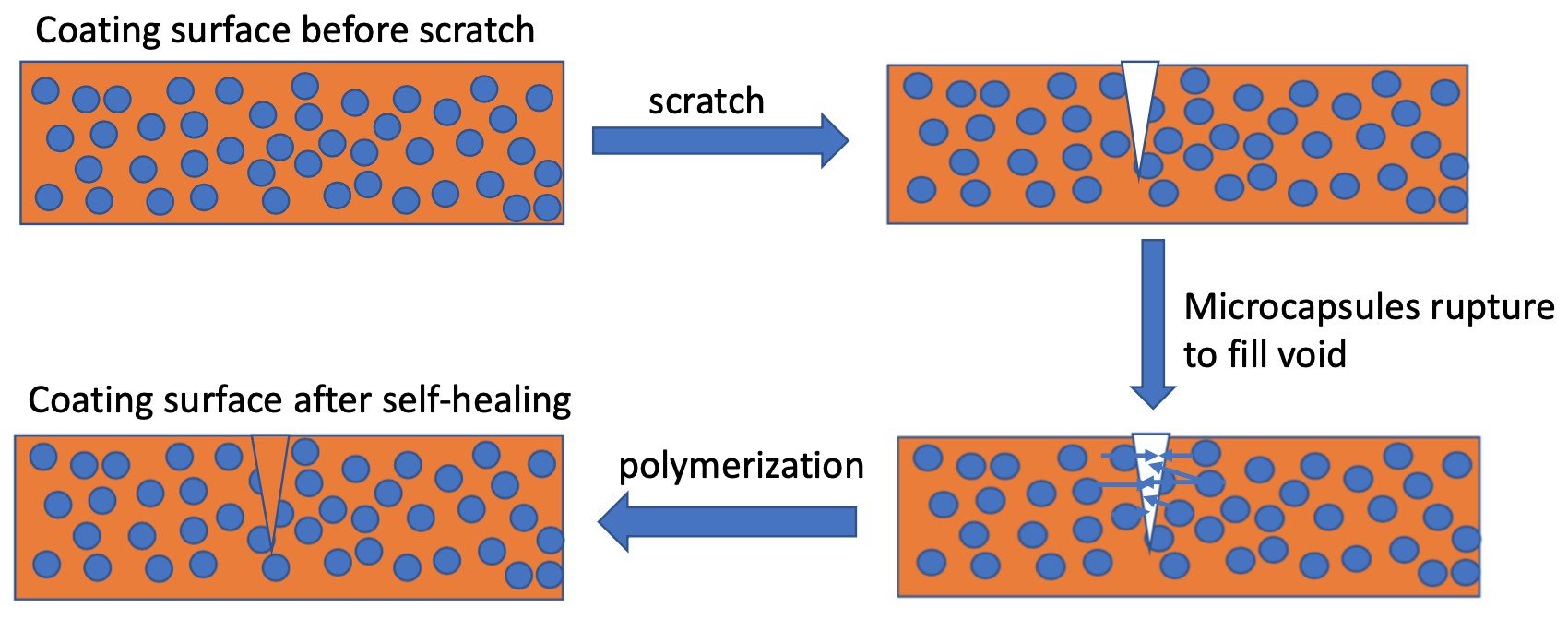
Seongpil An, et.al studied self-healing technology based on capsules or fibers. Once the coating is scratched, micro or nano-capsules containing catalyzed liquid polymerizable materials (e.g. drying oils, dicyclopentadiene) are released into the scratch. Figure 2 illustrates Self-Healing technology based on capsules or fibers. Once the capsules are ruptured, polymerization takes place filling the void and functions to reduce moisture ingress and thus improve corrosion resistance as well as the appearance of the coating. Fibers based on thermoplastic poly(e-caprolactone) distributed in an epoxy matrix is one example of self-healing technology to restore film integrity when exposed to heat.
Figure 2- Self Healing Coatings based on Capsules or Fibers
Environmentally sensing coatings
Able to respond to a change in their environment, these coatings have utility for multiple applications. For example some waterborne interior house paints contain a dye that changes color due to exposure to interior light or a change in pH during the drying process. Upon drying, the change in color from for example pink or purple helps to signify sufficient coverage over a similarly colored undercoat.
Coatings that contain a pH sensitive dye and fluorescent molecules are also used to detect corrosion. Another approach is the use of a Rhodamine B-based dopant in epoxy coatings to sense corrosion on both steel and aluminum as it responds to both a decrease in pH and the presence of Fe+++ ions.
Another fast growing area of smart coatings is the use of coatings that are modified to resist colonization of surfaces by viruses or bacteria. Most surfaces contain minute amounts of nutrients such as sugars, oils or phosphorous that serve to enable microbes to grow and reproduce.
Need help finding coatings?
Prospector can help speed along your research with technical datasheets and access to global equipment suppliers.
Search coatings here
Antimicrobial coatings
Antimicrobial coatings have utility in multiple applications including hospitals, kitchens, public bathrooms, transportation (taxi cabs, Uber vehicles, airplanes) and on hand rails and door knobs. Additives that have been successfully used include materials containing silver in various binders or absorbed onto a porous surface to enable slow release and improve longevity. Quaternary ammonium salts also provide antimicrobial activity, Quaternary ammonium salts can be more effective against viruses and fungi. Copper also provides some antimicrobial activity as well as organic based anti-bacterials such as Triclosan.
Table 1 – Summary of other Smart Coating Applications
| Coating Type | Principal | Stimulus | Smart Response |
| Solar Reflective | Reflect IR EnergyLight colors and dark colors using doped mixed metal oxides | Sunshine | Provides cooler surface, saves air conditioning cost |
| Piezoelectric | Pigment generates electrical current when stressed(Pb-Zr-Titanate) | Vibration | Creates an voltage when subjected to mechanical stress |
| Piezomagnetic | Polycrystalline materials generate magnetic field when stressed | Vibration | Creates a magnetic field when subjected to mechanical stress |
| Thermochromic | Change color in response to temperature liquid crystals and Leuco dye | Temperature | Indicates temperature change in a designated range |
| Electrochromic | Polymeric electrolyte that changes color when exposed to an electric current | Electric current | Color change, aesthetic appeal, indicator |
| Hydrophobic/hydrophilic | Surface modification coupled with adjusting surface tension | Moisture | Adjust water contact angle to repel (hydrophobic) or attract moisture (hydrophilic) |
For additional information concerning the selection of materials to enhance hydrophobicity, please navigate to www.ulprospector.com (EU).
- Organic Coatings, Science and Technology, Frank N. Jones et.al., Wiley & Sons, 2017
- PCI Magazine
- Science Direct
- Shape Memory Assisted Self- Healing Coatings, 2013, Material Science, Luo and Mather
- Transparency Market Research: Smart Coatings Market – Global Industry Analysis, Size, Share, Growth, Trends, and Forecast 2017-2025
- Seongpil An, Min Wook Lee, Alexander L. Yarin, Sam S. Yoon, A review on corrosion-protective extrinsic self-healing: Comparison of microcapsule-based systems and those based on core-shell vascular networks, Chemical Engineering Journal, Volume 344, 2018, Pages 206-220, ISSN 1385-8947, https://doi.org/10.1016/j.cej.2018.03.040.
The views, opinions and technical analyses presented here are those of the author or advertiser, and are not necessarily those of UL’s Prospector.com or UL LLC. All content is subject to copyright and may not be reproduced without prior authorization from UL or the advertiser. While the editors of this site may verify the accuracy of its content from time to time, we assume no responsibility for errors made by the author, editorial staff or any other contributor.